
The first-of-its-kind AI-driven, non-blood-contact smart closed-loop diagnostic and treatment platform for heart conditions
Integrating physiological sensing, biomechanical support, and molecularly targeted intervention into a single implantable platform redefines the diagnosis, monitoring, and treatment of heart failure and structural heart disease
Hydraulic Tech
Hollow tubes filled with tailored liquids
Customised Design
Gentle pressure can massage the ventricles
Advanced Cardio Care
Naturally conforms to the surface of the heart, providing effective support
Product Development and Scientific Validation: Active Soft Robotic Heart Support Device
From Concept to Evidence: A Solid Foundation of Preclinical Research
Our confidence in the ASD platform is grounded in extensive experimental data. Across multiple animal models, the ASD system has demonstrated multifunctional therapeutic benefits:
Mechanical Support Effectiveness: In rodent models, ASD demonstrated the ability to improve cardiac output and reverse adverse ventricular remodeling。
Advantages in Targeted Drug Delivery: Epicardial drug delivery via the ASD showed significantly higher bioavailability and therapeutic efficacy compared with intravenous administration (e.g., lidocaine for anti-arrhythmia, Danshen injection for heart failure therapy).
A Carrier for Innovative Therapies: We have successfully delivered novel therapeutic agents — such as photosynthetic microorganisms and stem cells — through the ASD, opening new avenues for future treatment paradigms.
These findings have been published in multiple peer-reviewed journals, providing a strong scientific foundation for advancing ASD technology from the laboratory towards clinical application.
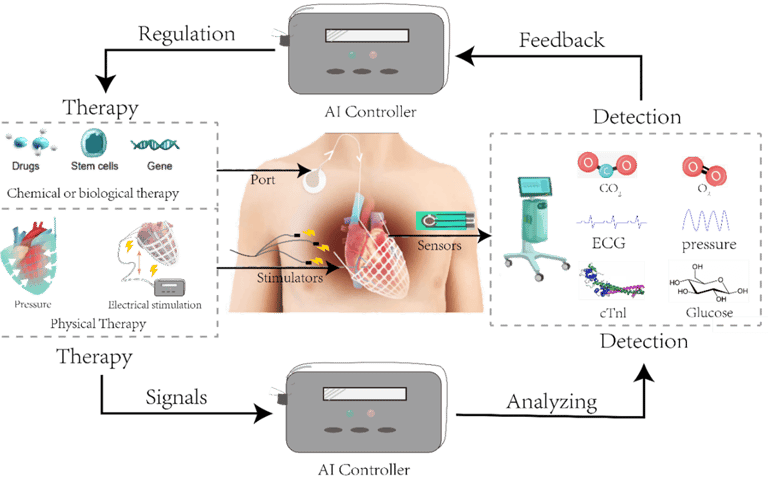
Active Soft Robotic Cardiac Support Device (ASD): Product Development Roadmap
We have formulated a clear strategic path to bring the ASD platform to clinical and market applications:
Phase I: Preclinical Development & System Optimisation (2026 – 2027)
Milestones: Completion of large-animal studies; identification of GMP manufacturing partners; integration of core sensing and algorithm modules.
Use of Funds: Team expansion, prototype iteration, regulatory consultation.
Phase II: First-in-Human Study (2027–2028)
Milestones: Initiation and completion of the first human feasibility study, validating safety and preliminary efficacy.
Phase III: Pivotal Clinical Trials & Market Preparation (2029–2031+)
Milestones: Launch of pivotal clinical trials, submission of regulatory applications, and commercial launch preparation.
Partnerships & Investment: We are seeking mission-aligned partners to advance this transformative cardiac recovery technology together.
Investors: Join us in supporting a platform technology with a strong IP portfolio, a clear development pathway, and significant global market potential.
Strategic Collaborators: Partner with us in areas such as sensing technology, AI algorithms, novel biologics, or clinical development.
Top Talents: Join our passionate, interdisciplinary team to tackle the most challenging clinical problems in heart failure.
Stay Updated
Get the latest on our cardiac device progress
Contact
Reach out for support or enquiries
Email:
Phone: +44 1223 981791
info@cardio-matrix.com
© 2025. Cambridge CardioMatrix, All rights reserved.
Find us at the heart of Cambridge, where innovation meets care in every beat
Cambridge CardioMatrix - MedTech International, Allia FBC, Kings Hedges Road, CB4 2HY Cambridge, UK
HOME ABOUT TECHNOLOGY DEVELOPMENT NEWS CONTACT US
